Triterpenoids — Structure, Food Sources, Benefits, and Side Effects
Introduction
Triterpenoids are a large and diverse class of phytochemicals—compounds produced by plants, usually for protective and developmental purposes. They also have potential uses in the food and pharmaceutical industries.
Triterpenoids are oxygen-containing derivatives or degradation products of the naturally occurring compound triterpene. However, sometimes the terms triterpenoid and triterpene are used interchangeably.
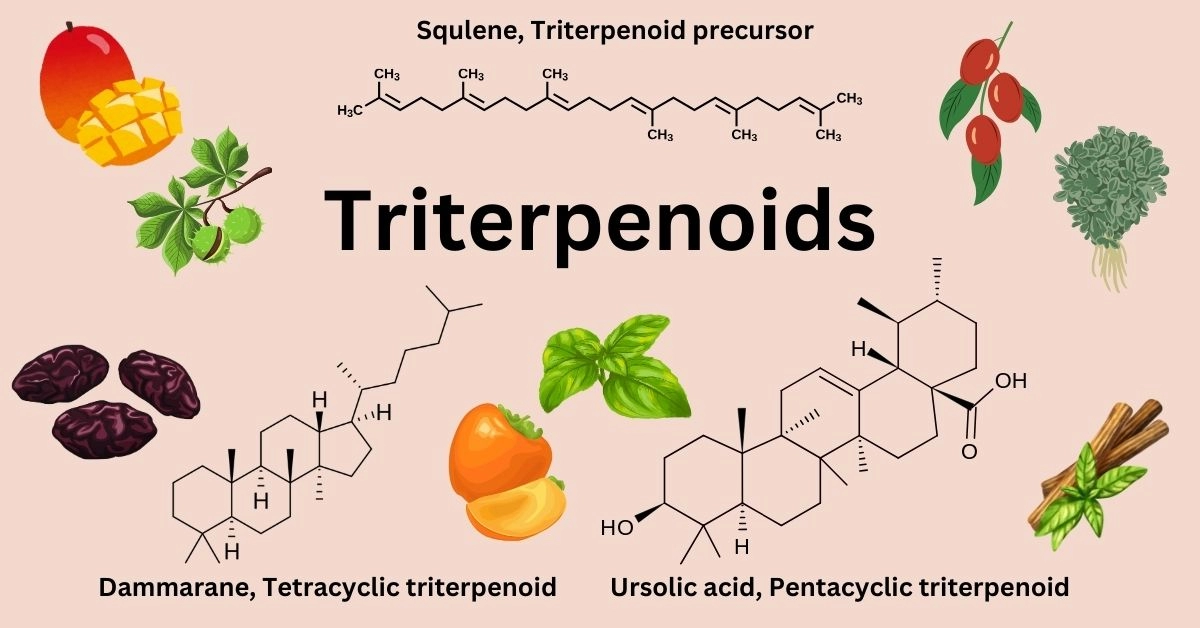
One of the most important triterpenoids is squalene, from which other triterpenoids are synthesized. However, squalene is also produced in animals and serves as a precursor to all steroids, including cholesterol.
In this article, we will present the available scientific evidence about triterpenoids and discuss their chemical properties, functions, and health benefits.
Triterpenoid Structure
Triterpenoids (C30H48), also known as isoprenoids, are a vast group of natural compounds originating from 30-carbon (C30) precursors. There are over 100 different structures within this group. They naturally occur in plants either in their free form or as glycosides or esters (1).
Small carbon fragments can be removed in the final stages of biosynthesis, which creates molecules with fewer than 30 carbon atoms, such as C27 steroids (2).
Most triterpenoid structures feature four or five rings, but there are also triterpenoids with no rings, as well as those with one, two, three, or even six rings, which have been discovered in nature. Based on the number of rings, most triterpenoids are divided into two groups - tetracyclic with four rings and pentacyclic with five.
Triterpenoids encompass various types of compounds, including triterpenes, steroids, limonoids, quassinoids, and both triterpenoidal and steroidal saponins (1).
To date, over 4,000 cyclic triterpenoids have been identified, including free triterpenoids and saponins with triterpenoid aglycones. These compounds are recognized for their wide range of biological activities (3).
The origin of most triterpenoids is the enzyme-driven cyclization of the compound called squalene or oxidosqualene. This set of biogenetic principles, which leads to the formation of triterpenoids, is coined as the isoprene rule (4).
Triterpenoids are derived from squalene, a natural triterpene with the chemical formula of C30H50. Squalene is also synthesized in animals and serves as a precursor for all steroids, including cholesterol.
Squalene, one of the most important triterpenes that serves as a precursor for other triterpenoids.
Oleanolic acid, an example of a pentacyclic triterpenoid.
Types of Triterpenoids
So far, more than 4000 individual triterpenoids have been documented in the literature. Most can be divided into two main groups based on the number of carbon rings - tetracyclic (4 rings) and pentacyclic (5 rings).
The major types of tetracyclic triterpenoids are protostane, dammarane, apotirucallane, lanostane, cycloartane, tirucallane, cucurbitane, fusidane, and euphane (5).
The pentacyclic triterpenoid group is the largest, consisting predominantly of six main categories: friedelane, lupane, ursane, oleanane, serratane, and taraxastane. Ursanes and oleananes, including compounds like oleanolic acid, ursolic acid, maslinic acid, uvaol, and erythrodiol, are the main triterpene structures found in plants, including many commonly consumed plant foods. Betulinic acid is another common triterpenoid and is a type of lupane (6).
Triterpenoid is a broad term, including several important subclasses:
- Triterpenes: These compounds serve as the basis for many complex natural substances.
- Limonoids: These are modified triterpenes commonly found in citrus fruits and some plants. They have various biological activities, including insecticidal properties.
- Quassinoids: Bitter compounds derived from triterpenes, found primarily in plants of the Simaroubaceae family. They are known for their medicinal properties.
- Triterpenoidal saponins: Glycosides derived from triterpenes, characterized by their soap-like properties. They have diverse biological activities, including antimicrobial and anti-inflammatory effects.
- Steroidal saponins: Glycosides derived from steroids have a similar soap-like property. They are found in various plants and have medicinal properties.
- Steroids: Derived from triterpenes, steroids have a characteristic fused ring structure. They include hormones, cholesterol, and certain vitamins. While these are synthesized mostly in animals, they are also produced in plants.
Food Sources Of Triterpenoids
Plants rich in triterpenoids come in a wide variety and are widely distributed worldwide. Some of these triterpenoid plant sources include mangos, fenugreek, holy basil, licorice, ginseng, horse chestnuts, Japanese persimmons, Indian jujube, and others (7).
Bitter gourd is a good source of cucurbitacin E, while figs and mangos are rich in lupeol. Licorice can contain high levels of glycyrrhizic acid, and monk fruit or luo han gou is rich in cucurbitanes (7, 8).
Dried fruits have also been shown to be good sources of triterpenoids. Raisins are rich in oleanolic acid, while dried cranberries, blueberries, and cherries are high in oleanolic and ursolic acids (9).
Plants such as holy basil, thyme, lavender, catnip, and peppermint leaves are also good sources of ursolic acid (7).
Limonoids are mostly found in citrus fruits. Horse chestnut can be used as a source of triterpenoid escin, while fenugreek is high in diosgenin (7).
Physiological Functions and Health Benefits
Extensive research on the synthesis and preclinical or clinical experiments of triterpenoids reveals that botanical triterpenoids and their derivatives are effective in treating inflammation, obesity, diabetes mellitus, atherosclerosis, and hyperlipidemia (elevated levels of fats in the blood). Additionally, they show tumor growth-inhibiting, liver-protective, antimicrobial, antifungal, pain-relieving, immune-modulating, and heart-strengthening effects (2, 8).
Various triterpenoids have different physiological effects on health. The table below summarizes research on animal models regarding the positive impacts of these triterpenoid compounds (8).
| Triterpenoid Compound | Disease |
| Arjunolic acid | Acute liver failure |
| Asiatic acid | Acute lung injury Cardiac hypertrophy Hemodynamic abnormality Sepsis Liver ischemia-reperfusion injury Parkinson’s disease |
| Betulin | Alcoholic liver disease Kidney injury |
| Betulinic acid | Diabetic kidney disease High blood glucose Cerebral ischemia-reperfusion injury Hepatocellular carcinoma (liver cancer) |
| Celastrol | Diabetic kidney injury Cardiac (or heart muscle) fibrosis Diabetic kidney disease Hepatocellular carcinoma Ischemic stroke Ulcerative colitis-related colorectal cancer Myocardial ischemia-reperfusion injury Kidney fibrosis Incisional pain Acute kidney injury |
| Corosolic acid | Atherosclerosis Insulin resistance |
| Cucurbitacin E | Liver fibrosis Central obesity |
| Echinocystic acid | Pain/depression dyad |
| Glycyrrhizic acid | Kidney injury Fatty liver disease Bronchial asthma Diabetic kidney disease |
| Lupeol | Inflammatory bowel disease |
| Maslinic acid | Cerebral ischemia-reperfusion injury Ischemic stroke Schizophrenia Cardiac hypertrophy Seizure Alcoholic liver disease |
| Oleanolic acid | Memory impairment Liver ischemia-reperfusion injury Hyperlipidemia Gut atrophy Cartilage degeneration Adiposity Postmenopausal osteoporosis Schizophrenia Colitis Liver injury Atherosclerosis Fatty tissue insulin resistance Depression Allergic asthma Hepatocellular carcinoma Diabetic kidney disease Cholestasis Chronic cyclosporin kidney disease |
| Pristimerin | Acute lung injury Autoimmune hepatitis Colon cancer Rheumatoid arthritis |
| Squalene | Ulcerative colitis |
| Ursolic acid | Lipid and glucose metabolism Liver cirrhosis Liver inflammation Chronic obstructive pulmonary disease Traumatic brain injury Ehrlich ascites carcinoma Fatty liver disease Allergic asthma Spinal cord injury Cerebral ischemia-reperfusion injury Abdominal aortic aneurysm Atherosclerosis Diabetic kidney disease Liver fibrosis |
Most of these effects are due to the antioxidant, anti-inflammatory, and antiapoptotic activities of triterpenoid compounds.
Clinical trials have researched the positive effects of triterpenoid ginsenoside on breast cancer, high blood pressure, and ischemic stroke. Similarly, CDDO has been studied in liver disease, glycyrrhizin in hepatitis C, betulinic acid in dysplastic nevus syndrome, and glycyrrhetinic acid in end-stage kidney disease (7).
Anti-Inflammatory Effects
NF-κB is an inflammatory factor that has been found to activate numerous genes that contribute to inflammatory diseases such as Alzheimer's, arthritis, and cancer.
Many triterpenoids from plants can be effective in reducing inflammation. These include compounds like amyrin, avicin, asiatic acid, astragaloside, betulin, betulinic acid, boswellic acid, celastrol, cucurbitacin, diosgenin, erythrodiol, escin, ganoderiol, ginsenosides, glycyrrhizin, glycyrrhetinic acid, gypenoside, lupeol, madecassic acid, maslinic acid, momordin, oleandrin, oleanolic acid, platycodon D, pristimerin, saikosaponins, ursolic acid, and withanolide. Many of these triterpenoids target NF-κB, resulting in its downregulation (7).
Other inflammatory pathways and factors that these triterpenoids inhibit include the anti-apoptotic proteins Bcl-2 and Bcl-xL, cell proliferator promoters COX-2, cyclins, invasive and metastatic genes ICAMs, angiogenic protein VEGF, as well as promoting pro-apoptotic caspases, PPARγ (7).
Anti Cancer Effects
Triterpenoids regulate factors such as NF-κB, TNF, and interleukins (IL-1β, IL-6, and IL-8), which act as key links between inflammation and cancer.
Triterpenoids have been found to inhibit tumor progression by activating the intrinsic apoptosis pathway. Many triterpenoids derived from spices, such as asiatic acid, astragaloside, celastrol, cucurbitacin, diosgenin, gypenoside, hederagenin, lupeol, and momordin, induce apoptosis in various cancer cells through multiple mechanisms. These triterpenoids commonly target the antiapoptotic protein Bcl-2, leading to programmed death in cancer cells (7).
The triterpenoid CDDO has been researched in clinical trials as a treatment option for solid tumors and lymphoid malignancies (7).
Depending on the dose, pentacyclic triterpenoids can induce anti-inflammatory, cell-protective, tumor-differentiating, proliferation-arresting, and apoptotic effects (7).
Depression, Anxiety, and Memory
Oral and intraperitoneal administration of betulinic acid, α/β-amyrin, and galphimine B has shown anti-anxiety activity in animals, and a pharmaceutical composition containing betulinic acid has been patented for the prevention or treatment of anxiety. A/β-amyrin has also been researched for its sedative effect in mice (6).
The α/β-amyrin has been shown to reduce immobility time in the behavioral despair test in mice. Another study found that the antidepressant action of β-amyrin palmitate might be due to the release of norepinephrine from newly synthesized pools (6).
Memory and Dementia
The administration of asiatic acid has been researched to improve passive avoidance memory and learning but did not affect active avoidance memory in animal studies (10).
Different tetracyclic triterpenoids, such as ginsenosides, ginkgolides, and cannabinoids, have been investigated for their potential as agents of neuroprotective effects against Alzheimer's disease. These compounds show encouraging biological activities both in laboratory studies (in vitro) and in animal models (in vivo), but they have yet to undergo clinical testing (6).
Other triterpenoids, including cornel iridoid glycoside, oleanolic acid, tenuifolin, cryptotanshinone, and ursolic acid, have also demonstrated significant neuroprotective effects in laboratory-based studies (6).
Triterpenoid Medications
Over the past century, numerous studies have explored the active compounds found in medicinal plants.
Ganoderma lucidum, rich in ganoderic acid, may prevent bronchitis, hepatitis, high blood pressure, arthritis, and nephritis. Antrodia cinnamomea, containing high levels of eburicoic acid, can help treat abdominal pain, itchy skin, intoxication, diarrhea, and inflammation.
Schisandra chinensis, as a source of schinenlactone can help protect from cough, fatigue, rheumatism, amnesia, contusions, insomnia, and arthritis (8).
The table below summarizes some of the triterpenoid-containing medication products and their indications.
| Products | Constituent | Indications |
| Asiaticosides | Centella | Trauma, surgical trauma, burns, keloids, and scleroderma |
| Compound centella tablet | Centella | It improves blood circulation, removes blood stasis, and relieves pain. It is used for falls, injuries, and limb pain. |
| Compound glycyrrhizin tablets | Glycyrrhizic acid | Chronic liver disease, improving liver function tests, eczema, dermatitis, alopecia areata |
| Extractum glycyrrhizae liquidum | Licorice | Bronchitis, pharyngitis, bronchial asthma, chronic adrenal insufficiency |
| Ganoderma lucidum nanogels | Ganoderic acid | Frostbite |
| Ganoderma capsule | Ganoderic acid | Insomnia, forgetfulness, physical weakness, neurasthenia |
| Fufang Lingzhi granules | Ganoderic acid | Acute hepatitis, chronic hepatitis |
| Oleanolic acid tablets | Oleanolic acid | Adjuvant therapy for acute and chronic hepatitis |
Products with ginseng are often indicated for palpitations, insomnia, “deficiency of qi and blood,” gastroparesis (stomach weakness), nausea and vomiting, abdominal pain and loose stool, weakness, tiredness, and more (8).
Triterpenoid Toxicity
Some triterpenoids have therapeutic drawbacks because of their hemolytic (destroying red blood cells) and cytostatic (slowing the growth of cells) properties, which can cause toxic effects. Researchers are working on semisynthetic derivatives of triterpenoids to overcome these issues (2).
Some research on the toxicity of triterpenoids found the following side effects (8).
| Triterpenoid compound | Model | Side Effects |
| CDDO | Solid tumor | Pulmonary embolism |
| CDDO-Me | Chronic kidney disease Type 2 diabetes | Depending on the study
|
| CDDO-Me | Healthy adult | Abdominal pain, diarrhea, urinary tract infection, headache |
| Glycyrrhizic acid | Chronic hemodialysis | Diarrhea (minority) |
| Glycyrrhizic acid | Healthy adult | No adverse effects |
| Licorice root (glycyrrhizic acid) | Acute ischemic stroke | No adverse effects |
| Bitter melon (cucurbitacin E) | Type 2 diabetes | Headache and dizziness, nausea, vomiting, constipation |
History
In 1788, Lowitz initially extracted betulin from birch bark, but it wasn't until 1831 that Mason gave it its name. During the nineteenth century, researchers gradually isolated key triterpenoids such as oleanolic acid, ursolic acid, and glycyrrhetinic acid. In 1887, Vesterberg successfully isolated pure α- and β-amyrin and determined their molecular formulas (8).
Following these discoveries, scholars increasingly focused on the structural chemistry of triterpenoids. However, significant advancements in triterpenoid research were not made until the mid-twentieth century, when the isoprene rule theory was formed by Ruzicka in 1953 (8).
Summary
Triterpenoids (C30H48), also known as isoprenoids, are a vast group of natural compounds originating from 30-carbon (C30) precursors. There are over 100 different structures and more than 4000 individual triterpenoids within this group.
Most can be divided into two main groups based on the number of carbon rings - tetracyclic (4 rings) and pentacyclic (5 rings). The major types of tetracyclic triterpenoids are protostane, dammarane, apotirucallane, lanostane, cycloartane, tirucallane, cucurbitane, fusidane, and euphane. The pentacyclic triterpenoid group is the largest, consisting predominantly of six main categories: friedelane, lupane, ursane, oleanane, serratane, and taraxastane.
Some of these triterpenoid plant sources include mangos, fenugreek, holy basil, licorice, ginseng, horse chestnuts, Japanese persimmons, Indian jujube, raising, blueberries, cranberries, and others.
Extensive research on the synthesis and preclinical or clinical experiments of triterpenoids reveals that botanical triterpenoids and their derivatives are effective in treating inflammation, obesity, diabetes mellitus, atherosclerosis, and hyperlipidemia. Additionally, they exhibit tumor growth-inhibiting, liver-protective, antimicrobial, antifungal, pain-relieving, immune-modulating, and heart-strengthening effects.
Triterpenoids have also been researched to have neuroprotective and sedative effects that can help prevent or treat dementia, depression, and anxiety.
Triterpenoid-containing medicinal plants have been used to treat various diseases for decades. Today, medications synthesized or derived from these plants are increasingly utilized across a wide range of conditions. However, it's crucial to consider the potential toxicity and contraindications associated with their use.
References
- https://www.sciencedirect.com/science/article/abs/pii/B9780128179017000125
- https://www.sciencedirect.com/science/article/abs/pii/B9780128133743000077
- https://www.sciencedirect.com/science/article/abs/pii/B9780128194836000126
- https://www.sciencedirect.com/science/article/abs/pii/S0031942203006927
- https://www.researchgate.net/figure/Major-types-of-tetracyclic-triterpenoids_fig4_316911530
- https://www.researchgate.net/publication/236623047
- https://www.researchgate.net/publication/45200877
- https://www.researchgate.net/publication/353774933
- https://www.jstage.jst.go.jp/article/fstr/19/1/19_113/_pdf
- https://pubmed.ncbi.nlm.nih.gov/21167268/
