Gallic Acid — Structure, Health Benefits, Food Sources, and Toxicity
Introduction
Gallic acid or gallate is a naturally occurring organic acid found in various plants, fruits, and foods. It is a type of phenolic acid with the chemical formula C₇H₆O₅. Gallic is said to have various health-beneficial effects.
In this article, we will explore gallic acid, its chemical structure, the foods it is commonly found in, and its impact on our health.
Table of contents
Structure, Properties, and Types
Gallic acid is a phytochemical, a biologically active natural compound found in plant-based foods, first identified in 1786 by the scientist Carl Wilhelm Scheele. It belongs to a class of phytochemicals known as phenols or phenolic compounds, and more specifically, it is categorized as a phenolic acid (1).
Phenols are characterized by one or more hydroxyl (-OH) groups attached to an aromatic ring. Gallic acid is one of the most abundant phenolic acids in the plant kingdom (2).
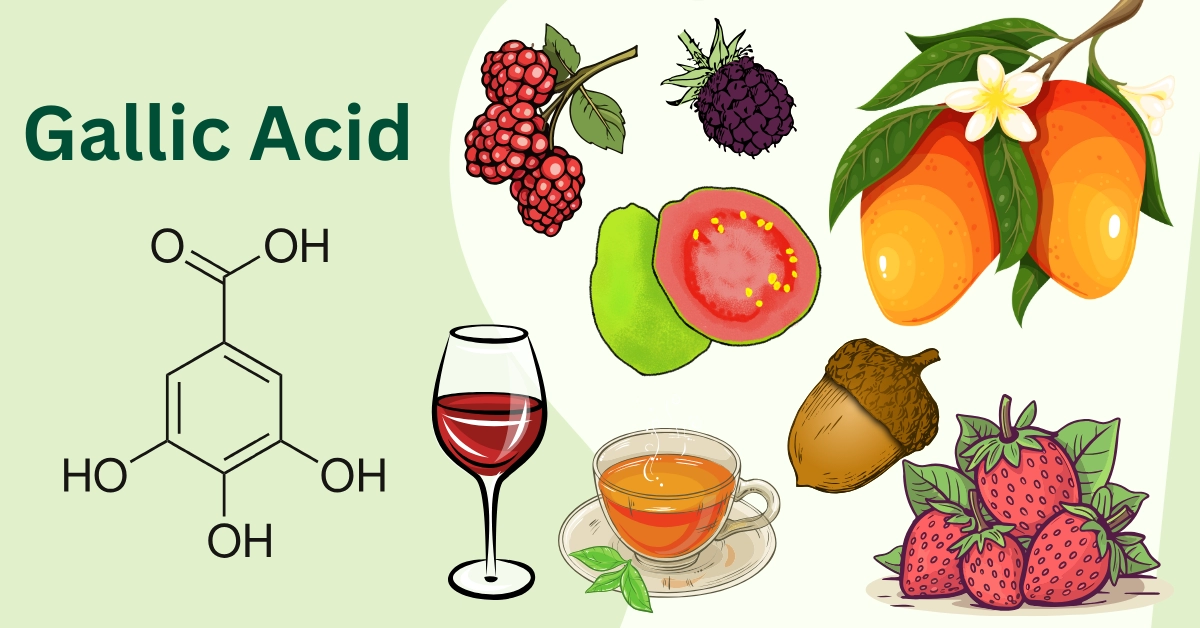
Gallic acid, also known as 3,4,5-trihydroxybenzoic acid (C6H2(OH)3COOH).
Gallic acid is a crystalline chemical compound that is odorless, usually colorless or slightly yellow. Its melting point is 210°C, and its decomposition point is 235 to 240°C. It is soluble in water, alcohol, and glycerol (1).
Gallic acid is produced through the shikimic acid or phenylpropanoid pathway, an essential pathway in the synthesis of secondary metabolites with aromatic structures. However, from an industrial point of view, it is usually produced by breaking down tannic acid with the use of hydrolytic enzymes, such as tannase.
Derivatives of gallic acid include lauryl gallate, propyl gallate, octyl gallate, tetradecyl gallate, and hexadecyl gallate (1). Gallates are salt and ester derivatives of gallic acid.
Food Sources Of Gallic Acid
Gallic acid and its derivatives naturally occur in almost all plant parts, such as bark, wood, leaves, fruits, roots, and seeds. These compounds are found in different amounts in various common foods, including blueberries, blackberries, strawberries, plums, grapes, mangoes, cashew nuts, hazelnuts, walnuts, tea, wine, and more (3).
In the free state, gallic acid is found in many plant foods. As such, it may be regarded as the primary dietary polyphenol. Gallate concentrations differ depending on the type of plant species, as well as within a plant species, in response to various environmental elements like UV rays, microbial diseases, insect attacks, and chemical stressors.
Gooseberries, black and red currants, strawberries, raspberries, blueberries, and other red fruits are good providers of gallic acid. Furthermore, free-form gallate has been detected in red and white wine, with concentrations ranging from a few mg to several tens of mg per glass, as well as in common type and summer grapes (up to 4 g/kg fresh weight of phenolic compounds) (3).
Both green and black teas have gallic acid; however, the average amount in black teas (125 mg/L) is higher than in green teas (6 mg/L). Additionally, gallate can be found in oat flour (Avena sativa) and certain rice cultivars (Oryza sativa L.), with amounts ranging from 17 to 35 mg/Kg (3).
Below, you can find a list of foods and their gallic acid content according to several sources (4, 5).
Food Name | Gallic Acid Content |
19 to 102 mg/kg | |
21 to 89 mg/kg | |
Grape juice (black) | 79mg/kg |
Grape juice (green) | 110mg/kg |
8 to 67 mg/kg | |
Black currant | 30 to 62 mg/kg |
3 to 38 mg/kg | |
1 to 5 mg/kg | |
Evening primrose | 15 to 36 mg/kg |
Wild licorice root | 32mg/kg |
4.2mg/100g* | |
7.3 to 23.9 mg/100g* | |
0.5 to 2.5 mg/100g* | |
94.6 to 98.7 mg/100g* | |
Pomegranate juice | 4 to 14 mg/100g* |
* Gallic acid concentration mg/100g dry weight
Physiological Functions and Health Benefits
Gallic acid offers numerous health benefits due to its potent antioxidant, anti-inflammatory, and antimicrobial properties. It helps protect cells from oxidative damage, reducing the risk of chronic diseases such as cancer and cardiovascular conditions. Additionally, gallic acid inhibits the growth of harmful bacteria and enhances the antimicrobial activity of many antibiotics.
Anti-Inflammatory Effects
Gallic acid is linked to several mechanisms that reduce proinflammatory factors in the human body. Gallic acid can decrease the expression and activity of enzymes like inducible nitric oxide synthase and myeloperoxidase, which are key players in inflammation. Additionally, gallic acid regulates pro-angiogenesis factors, supports the formation of new blood vessels, and modulates programmed cell death (4).
Research indicates that gallic acid inhibits the release of lipopolysaccharide-induced nitric oxide, prostaglandin E2, interleukin-6, and cyclooxygenase-2 from macrophages during inflammation. It has also been demonstrated that gallic acid exerts anti-inflammatory effects by inhibiting the NF-κB pathway, a key pro-inflammatory signaling route in the body (4).
Gallates can improve the symptoms of allergic inflammatory diseases by reducing immune responses by regulating inflammatory pathways like MAPK, NF-κB, and MyD88 (6).
Gallic acid has also been researched as a potential treatment option for rheumatoid arthritis due to its strong anti-inflammatory effects and inhibitory effect on the pathological multiplication of fibroblasts (6).
Anti Cancer Effects
Gallic acid has demonstrated cytotoxic and antitumor effects by modulating the balance between antioxidants and pro-oxidants. In certain instances, it manages reactive oxygen species (ROS)-induced cancer formation by enhancing the activity of enzymes such as superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase, as well as by reducing lipid peroxidation and ROS production. In other cases, gallic acid can induce cell cycle arrest, autophagy, and programmed cell death by activating the caspase pathway and generating ROS. Additionally, it can prevent invasion and metastasis by decreasing the expression and activity of matrix metalloproteinases (4).
Gallic acid has been found to partially suppress the PI3K/Akt pathway, an important signaling route that regulates the activation and expression of various cancer-related factors. Research finds that gallate has anticancer effects in various cancer cell types, including lung cancer, bladder cancer, myeloid leukemia, and metastatic breast cancer (7).
Additionally, gallic acid has demonstrated tumor-suppressive effects in osteosarcoma, hepatocellular carcinoma, and precancerous regions of the stomach by regulating β-catenin (7).
Gallic acid's anticancer properties have also been researched for prostate, melanoma, and colon cancer (8).
Anti-Microbial Effects
Gallic acid can inhibit the motility, adherence, and biofilm formation of bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, Chromobacterium violaceum, and Listeria monocytogenes. Additionally, it can disrupt the integrity of cell membranes in both Gram-positive and Gram-negative bacteria, altering their charge, hydrophobicity, and permeability. In Campylobacter jejuni, gallic acid can affect membrane permeability and increase antibiotic accumulation within the microorganism (4).
Gallic acid, alkyl gallates, and chitosan-based forms of gallic acid can enhance the antimicrobial activity of various antibiotics, including erythromycin, gentamicin, norfloxacin, ciprofloxacin, ampicillin, penicillin, oxacillin, and tulathromycin, through synergistic effects (4, 9, 10).
Neuroprotective Effects
Gallic acid and its derivatives are recognized as natural antioxidants targeting various molecular pathways. Research presents evidence on the neuroprotective effects of gallate and its derivatives, highlighting their actions against neuroinflammation, neurodegeneration, and neurotoxicity in neurological diseases, such as multiple sclerosis (MS), ischemic stroke, Alzheimer’s disease, and Parkinson’s disease (3).
Gastrointestinal Health
Studies have shown that due to its anti-inflammatory properties, gallic acid, in a dose-dependent manner, lowers the disease activity index and mitigates both macroscopic and microscopic damage, such as alterations in the mucous membranes of the colon and stomach in diseases like ulcerative colitis and Crohn’s disease (4, 6).
Cardiovascular Health and Metabolic Disease
Gallic acid pretreatment reduces the oxidative damage from myocardial infarction due to its antioxidant properties. It boosts the activity of antioxidant enzymes like SOD, CAT, GST, and GPx, and increases levels of non-enzymatic antioxidants such as GSH, vitamin C, and vitamin E. These actions help protect myocyte membranes from free radical damage, resulting in lower levels of serum cardiac biomarkers like cardiac troponin T (cTnT) and creatine kinase-MB (CK-MB) after infarction (2).
Gallic acid can help in metabolic disorders by inhibiting diet-induced high blood sugar and triglycerides, reducing fat cell size, and protecting pancreatic β-cells. It induces the expression of PPAR-γ, a transcription factor that promotes differentiation and insulin sensitivity in adipocytes. Additionally, gallic acid enhances cellular glucose uptake (2).
Gallic Acid Toxicity
Gallic acid has been found to be generally safe and effective for most cells at lower concentrations, but it becomes toxic at relatively higher concentrations (5).
In an acute oral toxicity study, no signs of lethal toxicity were observed at a dose of 5000 mg/kg. In a subacute toxicity study, a dose of 1000 mg/kg was found to be non-toxic, demonstrating the safety of gallic acid (11).
Summary
Gallic acid or gallate is one of the most abundant phenolic acids in the plant kingdom, with a chemical formula of C₇H₆O₅. Derivatives of gallic acid include lauryl gallate, propyl gallate, octyl gallate, tetradecyl gallate, and hexadecyl gallate.
Gallic acid and its derivatives naturally occur in almost all plant parts, such as bark, wood, leaves, fruits, roots, and seeds. These compounds are found in different amounts in a variety of common foods, including blueberries, blackberries, strawberries, plums, grapes, mangoes, cashew nuts, hazelnuts, walnuts, tea, wine, and more.
Gallic acid has been researched to have numerous health benefits due to its potent antioxidant, anti-inflammatory, and antimicrobial properties. It helps protect cells from oxidative damage, reducing the risk of chronic diseases such as cancer, neurodegenerative, and cardiovascular conditions. Additionally, gallic acid inhibits the growth of harmful bacteria and enhances the antimicrobial activity of many antibiotics.
References
- https://www.researchgate.net/publication/282760267
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6528712/
- https://pubmed.ncbi.nlm.nih.gov/24938889
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9919014/
- https://www.researchgate.net/figure/Some-plants-containing-gallic-acid-on-the-basis-of-data-from_fig1_354399935
- https://www.sciencedirect.com/science/article/pii/S075333222031177X
- https://www.mdpi.com/2072-6643/15/16/3596
- https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.3615
- https://www.sciencedirect.com/science/article/abs/pii/S08824010220043
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6328469/
- https://www.sciencedirect.com/science/article/abs/pii/B9780128243152011891
