Iron — Health Benefits, Nutrition Sources, Deficiency Symptoms
Introduction
Iron is not only a prevalent mineral on Earth but also a fundamental micronutrient for most living organisms, crucial for maintaining normal physiological functions.
In this text, we explore the importance of iron in the human body, its sources, absorption, impact on overall well-being, and what happens to the body when there’s not enough or too much of it.
Table of contents
- Recommended Intake
- Food Sources Of Iron
- Iron Supplements
- Absorption, Metabolism, and Regulation
- Iron Absorption Factors
- Health Benefits and Other Functions
- Oxygen Transport
- Energy Production
- DNA Synthesis and Repair
- Neurotransmitter Synthesis
- Immune Function
- Muscle Function
- Iron Deficiency
- Iron Toxicity
- Interactions with Medications
- Summary
Recommended Intake
The recommended dietary allowance (RDA) for iron varies depending on age, gender, physiological state, and even diet. The table below shows the RDA for iron based on age and gender (1).
| 2-3 | 4-8 | 9-13 | 14-18 | 19-30 | 31-50 | 51+ | |
| Male | 7 | 10 | 8 | 11 | 8 | 8 | 8 |
| Female | 7 | 10 | 8 | 15 | 18 | 18 | 8 |
As can be seen in the chart, women in their reproductive years require significantly more iron due to its monthly loss during menstruation.
The recommended daily iron intake is 1.8 times higher for vegetarians than those who consume meat. This difference arises because heme iron, found predominantly in meat, offers greater bioavailability than nonheme iron from plant-based foods. Additionally, meat, poultry, and seafood consumption enhances the absorption of nonheme iron (2).
Iron plays a crucial role as a nutrient during pregnancy, supporting fetal development. Approximately 1 in 10 pregnant women and 1 in 4 women in their third trimester experience iron deficiency. Pregnant women in all trimesters are recommended to have 27mg of iron per day, while the RDA for lactating women over the age of 18 is 9 (1).
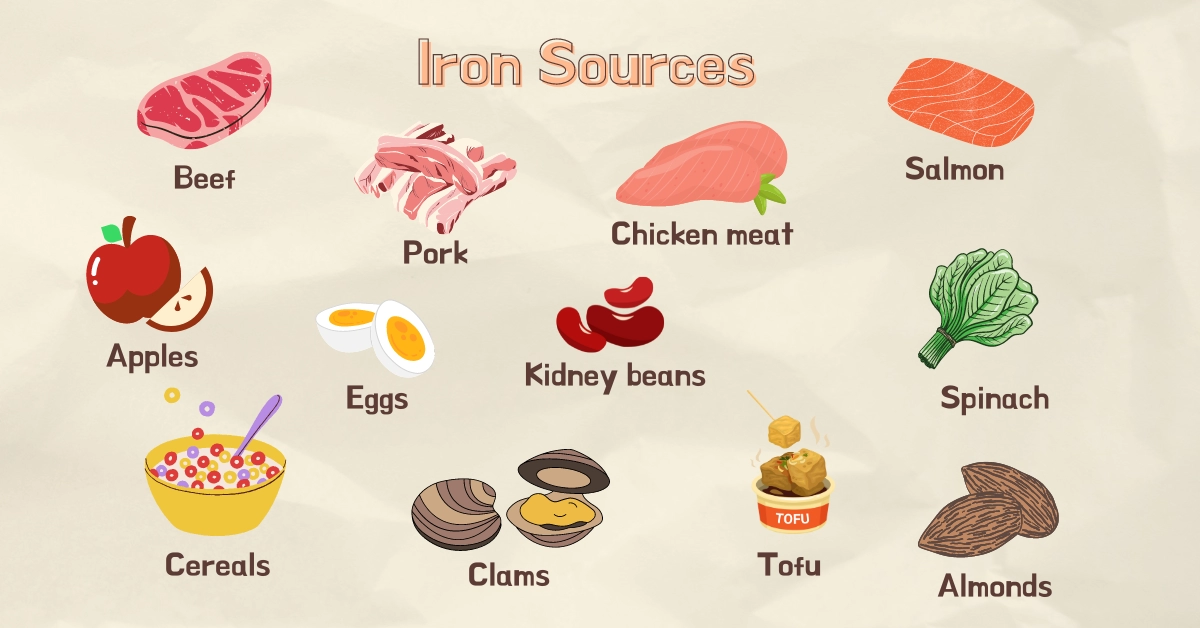
Food Sources Of Iron
The main food groups rich in iron are meat products, seafood, beans and lentils, certain fruits, leafy greens, whole grains, fortified cereals, and nuts.
Red meat tends to be higher in iron compared to white meat, as myoglobin is an iron-containing protein that gives meat its dark red hue.
Below, you can find a table with a few iron-rich foods based on average serving sizes per person.
| Food Name | Serving Size | Iron Content |
| Beef (roisted tenderloin) | 85g (3 ounces) | 2.6mg |
| Pork loin (broiled) | 85g (3 ounces) | 0.74mg |
| Pork shoulder (braised) | 85g (3 ounces) | 1.5mg |
| Chicken (roasted, with meat and skin) | 85g (3 ounces) | 1.1mg |
| Salmon (Atlantic, dry heated) | 85g (3 ounces) | 0.29mg |
| Apples (raw, with skin) | 125g (1 cup, chopped) | 0.15mg |
| Eggs (hard-boiled) | 50g (1 large) | 0.6mg |
| Kidney beans (boiled) | 89g (0.5 cup) | 2mg |
| Tofu (raw) | 81g (0.25 block) | 2.2mg |
| Clam | 85g (3 ounces) | 2.4mg |
| Almond | 28.35g (23 whole kernels) | 1.05mg |
| Cereal (ready-to-eat) | 29g (1 cup) | 9.9mg |
| Spinach (boiled) | 180g (1 cup) | 6.4mg |
On our page, you can also find a list of foods high in iron based on 100g servings.
Iron Supplements
Iron supplements are oral medications that contain iron, commonly used to treat iron deficiency anemia. Iron supplements are available over the counter or by prescription and come in various forms, including ferrous sulfate, ferrous gluconate, and ferrous fumarate.
The dosage of iron supplements depends on the severity of iron deficiency and individual factors such as age and underlying health conditions. It is crucial to follow healthcare provider instructions regarding dosage and duration of supplementation.
Iron supplements are often recommended to be taken on an empty stomach to enhance absorption, although some individuals may experience less gastrointestinal discomfort when taken with food. It is best to take iron supplements in the morning with orange juice (3).
However, these supplements can have side effects, including bloating, constipation, diarrhea, nausea, vomiting, stomach cramps, and darkening of stools.
Iron supplements should not be taken with milk, calcium, antacids, high-fiber foods, or caffeine (4).
Absorption, Metabolism, and Regulation
Iron absorption primarily occurs in the duodenum and upper small intestine. After a few chemical changes, iron can be stored as the protein ferritin or absorbed into the bloodstream and transported predominantly to the bone marrow by a glycoprotein called transferrin. Here, the iron is used to produce red blood cells.
The body stores 3 to 4g of iron, most of which is found as part of the protein hemoglobin in red blood cells (5). The spleen, bone marrow, and liver recycle this iron, taking it from dying red blood cells and reusing it for new processes. Most of the remaining iron is stored in muscles as myoglobin or liver cells as ferritin or hemosiderin.
Liver cells or hepatocytes secrete a hormone called hepcidin, which plays a crucial role in regulating iron metabolism. High levels of iron, inflammatory compounds, or oxygen in the blood induce hepcidin excretion. In turn, hepcidin significantly reduces iron absorption and plasma iron levels by decreasing ferroportin, a protein that exports iron from intestinal cells into the blood. A feedback mechanism can also increase iron absorption when it’s deficient in the body.
Unlike most other micronutrients, iron levels are essentially only controlled through altered absorption (6).
Iron excretion is an unregulated process. Small amounts of iron are lost daily through sweat, menstruation, and shedding of hair, skin, and the mucosal lining of the gastrointestinal tract.
Iron Absorption Factors
Two forms of dietary iron exist - heme iron (found in animal sources) and non-heme iron (found in plant and animal sources). Heme iron is more readily absorbed than non-heme iron.
Plant-based foods often contain phytate, which decreases non-heme iron absorption. Additionally, antioxidant compound polyphenols in tea, coffee, red wine, some legumes, fruits, and vegetables can also inhibit non-heme iron absorption (6).
Calcium, some animal proteins, such as casein, and some plant proteins, such as soy protein, can lower both heme and non-heme iron absorption (6).
Oxalic acid found in plant-based foods also binds to iron and lowers absorption. If you are interested in the oxalate content of foods, you can visit this website.
Conversely, vitamin C or ascorbic acid increases iron absorption and can outweigh the negative effects of iron absorption inhibitors when used in a diet high in non-heme iron (6).
Health Benefits and Other Functions
Iron serves several critical functions in the human body. Below, we talk about some of the most important ones.
Oxygen Transport
Iron is a key component of hemoglobin, the protein in red blood cells responsible for carrying oxygen from the lungs to tissues throughout the body. Hemoglobin binds to oxygen in the lungs and releases it in tissues where it's needed for cellular respiration.
Energy Production
Iron is essential for the proper function of enzymes involved in energy metabolism, particularly those involved in the electron transport chain within mitochondria. These enzymes are responsible for generating ATP, the primary energy currency of cells.
DNA Synthesis and Repair
Iron is required for DNA synthesis and repair processes. It is involved in the activity of enzymes responsible for DNA replication, cell division, and repair of damaged DNA strands, thus supporting cellular growth and proliferation.
Neurotransmitter Synthesis
Iron plays a role in the synthesis of neurotransmitters such as dopamine, serotonin, and norepinephrine. These neurotransmitters are essential for proper brain function, mood regulation, and cognitive processes.
Immune Function
Iron is necessary for the function of immune cells, including lymphocytes and macrophages, which play vital roles in defending the body against infections and foreign invaders. Iron is involved in various immune processes, including the production of reactive oxygen species (ROS) by immune cells to kill pathogens. However, the same free radicals can damage healthy tissues and are the leading cause of iron toxicity, which will be discussed later.
Muscle Function
Myoglobin, a protein found in muscle cells, contains iron and stores and transports oxygen within muscle tissues. Myoglobin facilitates oxygen delivery to muscle cells during periods of increased activity or exercise.
Iron Deficiency
Iron deficiency is a state characterized by the absence of readily available iron reserves and the presence of indicators suggesting insufficient iron supply to tissues, including the red blood cell-producing system (7).
Iron deficiency causes 50% to 60% of anemia worldwide (8). It is said that 30% of the world's population is affected by iron deficiency anemia to some extent (9).
However, in some cases, iron deficiency can exist without anemia, as a considerable quantity of iron must be lost before hemoglobin levels decrease. Iron deficiency without anemia can have general symptoms, such as weakness and fatigue; however, most functional changes occur with iron deficiency anemia (IDA).
Iron Deficiency Anemia
Anemia is a medical condition characterized by a deficiency in the number or quality of red blood cells or a decreased amount of hemoglobin. Anemia is defined as blood hemoglobin levels below 130g/L in men and 120g/L in women.
As iron plays a key role in the structure of hemoglobin, its deficiency leads to smaller red blood cells with less hemoglobin. This form of anemia is called microcytic, which can be translated as small-celled.
Iron deficiency anemia (IDA) can cause impaired cognitive development, immune function, and decreased work capacity (7).
The impaired oxygen transport causes IDA symptoms, such as fatigue, weakness, paleness, shortness of breath, heart palpitations, fast heart rate, and cold hands and feet.
Compensatory mechanisms lead to decreased blood flow to internal organs. Lowered intestinal blood flow causes symptoms like nausea, abdominal pain, malabsorption, and weight loss, furthering iron deficiency.
Decreased oxygen levels in the blood flow to the brain can lead to headaches, vertigo, lethargy, and impaired cognitive functions (9).
Other neurological symptoms of iron deficiency are the restless leg syndrome and pica. The restless leg syndrome is the compulsion to move one’s legs while at rest due to reduced iron levels in the brain (10). Pica is characterized by persistent and compulsive cravings for non-nutritive substances, such as dirt, clay, chalk, ice, paper, or hair.
Rare symptoms of iron deficiency are glossitis or tongue inflammation and dysphagia or difficulty swallowing (10).
Iron Deficiency Causes
Due to increased iron demand, children, adolescents, and pregnant women are a high-risk group for iron deficiency. Similarly, in this group are women of reproductive age because of iron loss during menstruation.
In turn, iron deficiency is seen less commonly in adult men and postmenopausal women.
Iron deficiency anemia is caused by inadequate dietary intake, blood loss, increased iron demand, and some medical conditions, such as malabsorption, kidney disease, cancer, autoimmune diseases, and infections.
The leading causes of iron deficiency anemia commonly involve gastrointestinal diseases and menstruation in women, while reduced dietary iron intake also contributes significantly to the condition (9). Causes of gastrointestinal bleeding can be stomach ulcers, prolonged use or misuse of non-steroidal anti-inflammatory drugs, such as ibuprofen or aspirin, and more.
Some medical conditions can impair the body's ability to absorb iron from the diet. These conditions include celiac disease, Crohn's disease, ulcerative colitis (backwash ileitis), gastric atrophy, short bowel syndrome, gastric bypass surgery, and parasitic infections.
A common cause of iron deficiency anemia in developing countries can be malaria and hookworm.
Iron Toxicity
While iron is an essential mineral for basic physiological human functions, excess iron can be toxic and health-damaging.
Iron toxicity, also known as iron poisoning or iron overload, occurs during excessive iron accumulation in the body. This condition can have severe health consequences and may be caused due to various factors, such as accidental iron ingestion, certain genetic disorders, and repeated blood transfusions.
Accidental ingestion of iron supplements is a common cause, especially of those containing high doses of elemental iron, which can be toxic if ingested in large quantities, particularly by children. Accidental ingestion of iron by children younger than six years old has even led to cases of fatal poisoning (4).
Up to 20 mg/kg of iron is typically tolerated with mild gastrointestinal symptoms. Between 20 to 60 mg/kg is mild to moderately toxic, while over 60 mg/kg can lead to severe symptoms and circulatory collapse (4).
Hereditary hemochromatosis is a genetic disorder that causes the body to absorb too much iron from the diet. Over time, excess iron accumulates in organs such as the liver, pancreas, and heart, leading to organ damage and dysfunction.
Individuals requiring frequent blood transfusions, such as those with certain types of anemia or thalassemia, may develop iron overload over time due to the iron in transfused blood.
Iron toxicity can result in a range of symptoms, including nausea, vomiting, abdominal pain, diarrhea, and lethargy. In severe cases, it can lead to organ failure, coma, and even death.
Iron overload can particularly damage the heart, liver, and endocrine organs. Excess iron forms free hydroxyl radicals that cause damage to tissues through oxidative reactions with lipids, proteins, and nucleic acids (6).
Interactions with Medications
The mechanisms of iron absorption in the duodenum are pH-dependent. Thus, gastric acid production plays a key role in this process. Gastric acid inhibitors such as omeprazole and pantoprazole can significantly reduce iron absorption (6).
The use of iron supplements can decrease the effectiveness of Parkinson’s and restless leg syndrome medication levodopa, such as Sinemet and Stalevo. Similarly, it can reduce the effectiveness of levothyroxine, used to treat hypothyroidism, goiter, and thyroid cancer (2).
Iron can decrease the bioavailability of certain antibiotics, such as tetracyclines, ciprofloxacin, and penicillamines. Iron can also negatively impact the effect of the antihypertensive drug methyldopa (11).
As mentioned above, calcium intake can decrease iron absorption. To solve this issue, the two should be taken at different times.
Summary
Overall, iron is indispensable for various physiological processes in the human body, including oxygen transport, energy production, DNA synthesis, neurotransmitter function, immune response, and muscle function. Maintaining adequate iron levels is crucial for overall health and well-being.
The recommended daily allowance for iron is 8mg to 11mg for men and 8mg to 18mg for women, depending on age. The RDA for vegetarians is 1.8 times higher than those who consume meat.
Food sources rich in iron are beef, pork, chicken, salmon, apples, eggs, beans, tofu, fortified cereals, and others.
Iron from animal sources tends to be more absorbable than iron from plant sources. Tea, coffee, some legumes, fruits, vegetables, and dairy decrease iron absorption, while vitamin C increases it.
Iron deficiency is the cause of 50% to 60% of anemia in the world. General symptoms are fatigue, heart palpitations, nausea, abdominal pain, headaches, and cold hands and feet.
Iron supplements should not be taken with certain drugs, such as omeprazole, levodopa, levothyroxine, ciprofloxacin, and methyldopa.
References
- https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
- https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
- https://onlinelibrary.wiley.com/doi/full/10.1002/ajh.26987
- https://www.ncbi.nlm.nih.gov/books/NBK557376/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7611894
- https://www.ncbi.nlm.nih.gov/books/NBK448204/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3999603/
- https://jhoonline.biomedcentral.com/articles/10.1186/s13045-021-01202-2
- https://bmjopengastro.bmj.com/content/9/1/e000759
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3685880
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1368348/