Kaempferol — Structure, Rich Foods, Supplements, Benefits, and More
Summary
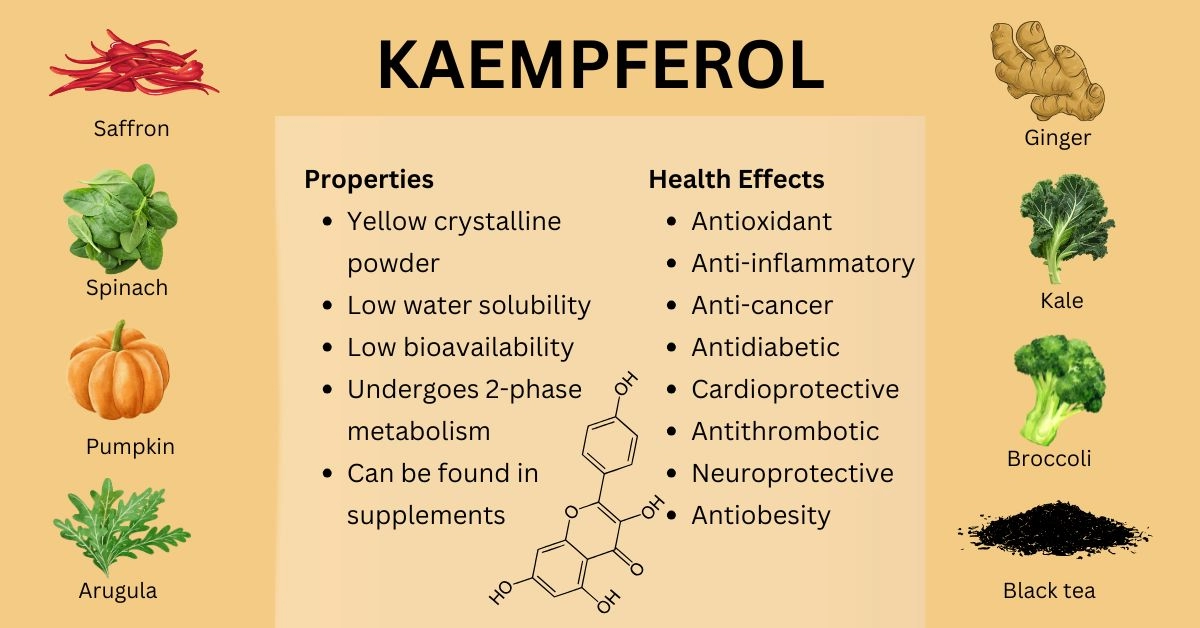
Kaempferol is one of the most studied flavonol-type flavonoids found in fruits, herbs, and vegetables, such as spinach, kale, broccoli, green onions, ginger, cauliflower, pumpkin, and black tea.
Due to its lipophilic properties and low water solubility, kaempferol has low oral bioavailability. Its metabolism involves 2 phases: phase I - oxidation and O-demethylation and phase II - sulfation, glucuronidation, and methylation. The most common metabolites of flavonoids circulating in the blood are methyl, sulfate, or glucuronide conjugates.
Like most dietary flavonoids, kaempferol has various health effects, including antioxidant, anti-inflammatory, anticancer, cardioprotective, antidiabetic, hepatoprotective, and neuroprotective effects. Kaempferol is best avoided during iron and folate deficiencies and etoposide (Vepesid) therapy.
Introduction
Kaempferol is one of the most studied flavonoids found in several fruits, vegetables, and herbs. It is named after Engelbert Kaempfer, a German doctor and a naturalist who traveled from Japan to the West and transported medical knowledge (1).
In this article, we’ll look into kaempferol’s classification, structure, dietary sources, health effects, and more.
Classification & Structure
Kaempferol is a flavonol, a type of flavonoid, which in turn is a polyphenol, a secondary plant metabolite.
Kaempferol is a pure yellow crystalline powder that melts at 276–278°C. Its molecular formula is C15H10O6, and its molecular weight is 286.23. It has four hydroxy groups located at positions 3, 5, 7, and 4' (tetrahydroxyflavone) (2, 3).
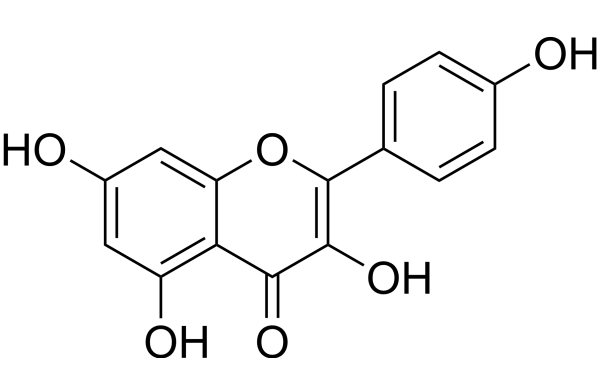
Kaempferol is soluble in hot ethanol and diethyl ether and has a slight water solubility (2, 3).
Absorption & Metabolism
Kaempferol is absorbed in the small intestine, primarily by passive diffusion, due to its lipophilic (fat-loving) nature. These properties and low water solubility lead to kaempferol having low oral bioavailability: the daily absorption is around 1–2g, and the plasma concentrations are in micromolar (μM) (3, 4).
Evidence suggests that some amounts can be absorbed through active transport, too. If kaempferol is bound to glucosides with many sugar molecules, it will travel to the large intestine, get fermented by the gut bacteria, and expose the glucose molecules for absorption.
After absorption, conjugated forms of kaempferol and some kaempferol glycosides are transported to the liver and metabolized. They are then distributed to body tissues and excreted by the urine. Kaempferol metabolism involves 2 phases: phase I - oxidation and O-demethylation and phase II - sulfation, glucuronidation, and methylation. The most common metabolites of flavonoids circulating in the blood are methyl, sulfate, or glucuronide conjugates, such as kaempferol-3-glucuronide (5, 6, 7).
Food Sources & Supplements
Kaempferol is widely distributed in herbs, fruits, and vegetables, such as spinach, kale, broccoli, green onions, ginger, cauliflower, pumpkin, and black tea.
The table below shows dietary kaempferol content in some foods, calculated per 100g fresh weight. The values may greatly change considering the average serving sizes of various foods (6, 7).
| Food/Beverage | Kaempferol content mg/100g |
| Capers | 259 |
| Saffron | 205 |
| Green onions (scallions) | 83.2 |
| Arugula | 59 |
| Spinach | 55 |
| Kale | 47 |
| Dill | 40 |
| Brown mustard | 38 |
| Pumpkin | 37.1 |
| Ginger | 34 |
| Cauliflower | 27 |
| Common beans | 26 |
| Chinese cabbage | 22.5 |
| Chives | 10 - 12.5 |
| Broccoli | 7.2 - 8 |
| Black tea | 1.7 - 11.8 per 100 mL |
| Cherry | 5.14 |
| Onions | 4.5 |
Some amounts of kaempferol are also found in endive, collard green, carrot, tomato, wild leek, Goji berry (dried), green chili, asparagus, lettuce, watercress, mustard greens, radish seed, turnip green, apple, watermelon, kiwi, apricot, peach, grape, grapefruit, citrus fruit, strawberry, blueberry, cranberry, red wine, and more (3, 8).
Kaempferol is also found in supplements, alone or with other phenolic compounds or molecules; the supplements are available in powder or capsule forms.
Health Impact
Like most dietary flavonoids, kaempferol possesses various health effects, such as antioxidant, anti-inflammatory, anticancer, cardioprotective, antidiabetic, and neuroprotective.
Antioxidant & Anti-inflammatory Effects
Antioxidants protect the cells by reducing cell damage caused by reactive oxygen species (ROS), lowering oxidative stress. Kaempferol may also increase macrophage levels, resulting in decreased tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) expression, as well as the expression of IL-6, IL-1β, and IL-18. As a result, the pro-inflammatory NF-κB signaling pathway is inhibited. Kaempferol also increases the expression of Nrf2-regulated genes and inhibits toll-like receptor 4 (TLR4).
Due to its antioxidant and anti-inflammatory effects, kaempferol has potential protective effects in various chronic inflammatory conditions, such as intervertebral disc degeneration, osteoarthritis, neurodegenerative disorders, cardiovascular diseases, autoimmune diseases, and mastitis (3, 9, 10).
Cancer
Kaempferol may decrease the risk of various cancers, such as esophageal cancer, breast cancer, cervical cancer, hepatocellular carcinoma, cholangiocarcinoma, ovarian cancer, stomach cancer, lung cancer (NSCLC), leukemia, bladder cancer, brain cancer, kidney cancer, osteosarcoma, pancreatic cancer, and uterine fibroids.
In vitro studies using human, rat, and mouse cell lines have shown that kaempferol has a wide range of cancer-targeting effects by modulating inflammation, apoptosis, angiogenesis, and metastasis. Additionally, kaempferol specifically targets cancer cell lines without affecting normal cells.
Kaempferol may promote apoptosis (programmed cell death), cause growth arrest, activate immunity, inhibit blood vessel growth in tumors, and lead to cell cycle arrest. In certain conditions, it may also act as a pro-oxidant, making cancer cells susceptible to copper-induced DNA breakage (3, 11, 12, 13).
Cardiovascular Health
Animal and in vitro studies have shown the cardioprotective effects of kaempferol, improving heart structure and function.
Kaempferol may protect against cardiotoxicity, sinus node dysfunction, arrhythmia, atherosclerosis, cardiac (heart) hypertrophy, and myocardial infusion/reperfusion injury, regulate myocardial calcium concentrations, apoptosis, and mitochondrial function, and inhibit vascular endothelial inflammation, and smooth muscle cell migration.
Additionally, kaempferol may inhibit thrombosis and platelet activation by reducing the enzymatic activities of thrombin and Factor X, fibrin polymer formation, and attenuating the phosphorylation of collagen/epinephrine- and thrombin-induced platelets (3, 14, 15, 16).
Liver Disease & Diabetes
The hepatoprotective or liver-protective properties of kaempferol are partially attributed to its antioxidant and anti-inflammatory properties.
Studies on animals and HepG2 cells have shown that kaempferol may reduce cellular damage, lipid peroxidation, oxidative stress, and cell death. It may also help restore glutathione levels, downregulate CYP2E1 expression and upregulate SIRT1 expression, and increase antioxidant activity in liver injury caused by different factors, such as alcohol, xenobiotics, and drugs.
Kaempferol may decrease hyaluronic acid, ALT, and AST, inhibit collagen synthesis, and activate HSC cells in liver fibrosis. Kaempferol may also decrease fat accumulation in the liver and viscera (belly fat), inhibit inflammation, and promote mitochondrial beta-oxidation in fatty liver (MASLD) (3, 17, 18).
During diabetes, kaempferol may improve insulin signaling and promote insulin secretion, reduce inflammation, protect pancreatic beta cells from the toxic effects of glucose, and improve various signaling pathways. Its antioxidant and anti-inflammatory effects reduce chronic inflammation and oxidative stress linked to diabetes progression (3, 4, 19).
Neurological Health
Kaempferol may reduce cognitive impairment, improve cognitive functions, protect against neurodegenerative disorders such as Parkinson’s and Alzheimer’s, and improve depression. It and its metabolites may also upregulate the expression of several proteins, reduce hippocampal oxidative stress and neuroinflammation, delay neurodegeneration, enhance Na+/K+-ATPase activity, and enhance neuronal survival and mitochondrial calcium channel activity.
In Parkinson’s disease, kaempferol may also improve dopamine levels and reduce neuronal loss in the substantia nigra, help restore tyrosine hydroxylase activity, and inhibit astrocyte proliferation, α-synuclein aggregation, and Lewy body formation.
As for Alzheimer's disease, kaempferol may also protect against glutamate- and β-amyloid accumulation-induced oxidative stress, inhibit Aβ deposition, and regulate apoptosis-related proteins (3, 20, 21).
Other Health Effects
- Animal models have demonstrated that kaempferol may inhibit the formation of hypertrophic scars (3).
- Kaempferol may regulate intestinal antioxidant function, intestinal inflammation, and intestinal barrier function, as well as help regulate gut microbiota (22).
- The natural flavonoid also shows antibacterial, antifungal, and antiprotozoal activities (23).
Risks & Contraindications of Kaempferol
While some researchers found kaempferol to have antimutagenic effects, others found it genotoxic. Depending on the chemical reaction, flavonoids may act as antioxidants and pro-oxidants; the latter plays a key role in kaempferol’s genotoxic effects.
According to in vitro studies, kaempferol has carcinogenic and toxic effects, while in vivo screenings, the same findings were not reported.
Some reports contraindicate kaempferol intake during folic acid (vitamin B9) and iron deficiency, which may reduce cellular uptake and bioavailability. The flavonoid is also best avoided in cancer patients on etoposide (Vepesid) therapy, suggesting kaempferol interferes with its bioavailability (11).
References
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9740324/
- https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6755486/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9490412/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3580176/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6835347/
- https://www.ars.usda.gov/ARSUserFiles/80400525/Articles/AICR03_VegFlav.pdf
- https://www.sciencedirect.com/science/article/pii/S0753332220312312
- https://www.sciencedirect.com/science/article/abs/pii/S1043466624003508
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7570692/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3601579/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC8470426/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC6631472/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC10255479/
- https://www.sciencedirect.com/science/article/pii/S001429992300626X
- https://pubmed.ncbi.nlm.nih.gov/26073152/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC9988374/
- https://www.tandfonline.com/doi/full/10.1080/13880209.2021.1877734
- https://www.mdpi.com/1424-8247/17/6/757
- https://www.sciencedirect.com/science/article/pii/S0753332223010065
- https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/cbf.3964
- https://www.mdpi.com/2076-3921/12/8/1642
- https://www.mdpi.com/1422-0067/23/23/15054
